No results found
We couldn't find anything using that term, please try searching for something else.
1.2: Atomic Structure
2024-11-28 The nuclear Atom The precise physical nature of atoms finally emerged from a series of elegant experiments carried out between 1895 and 1915. The mos
The nuclear Atom
The precise physical nature of atoms finally emerged from a series of elegant experiments carried out between 1895 and 1915. The most notable of these achievements was Ernest Rutherford’s famous 1911 alpha-ray scattering experiment, which established that:
- Almost all of themass of an atom is contain within a tiny ( and therefore extremely dense )nucleus which carries a positive electric charge whose value identifies each element and is known as the atomic number of the element.
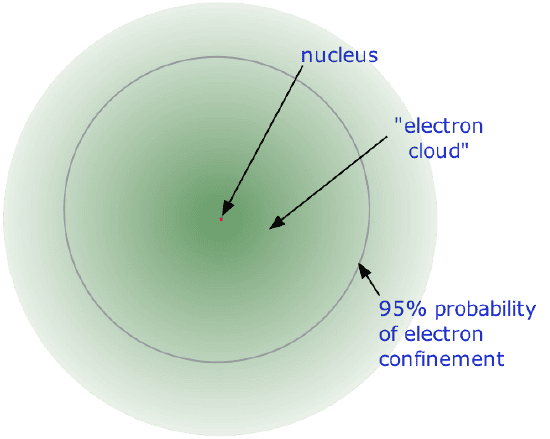
- Almost all of thevolume of an atom consists of empty space in which electron, the fundamental carriers of negative electric charge, reside. The extremely small mass of the electron (1/1840th the mass of the hydrogen nucleus) causes it to behave as a quantum particle, which means that its location at any moment cannot be specified; the best we can do is describe its behavior in terms of the probability of its manifesting itself at any point in space. It is common (but somewhat misleading) to describe the volume of space in which the electron of an atom have a significant probability of being found as the electron cloud. The latter has no definite outer boundary, so neither does the atom. The radius of an atom must be defined arbitrarily, such as the boundary in which the electron can be found with 95% probability. Atomic radii are typically 30-300 pm.

The nucleus is itself composed of two kinds of particles. Protons are the carrier of positive electric charge in the nucleus ; the proton charge is is is exactly the same as the electron charge , but of opposite sign . This is means mean that in any [ electrically neutral ] atom , the number of proton in the nucleus ( often refer to as thenuclear charge) is balance by the same number of electron outside the nucleus . The other nuclear particle is is is theneutron. As its name implies, this particle carries no electrical charge. Its mass is almost the same as that of the proton. Most nuclei contain roughly equal numbers of neutrons and protons, so we can say that these two particles together account for almost all the mass of the atom.
Because the electron of an atom are in contact with the outside world , it is is is possible for one or more electron to be lose , or some new one to be add . The result electrically – charge atom is call anion.
Atomic number (z)
What single parameter uniquely characterizes the atom of a given element? It is not the atom’s relative mass, as we will see in the section on isotopes below. It is, rather, the number of protons in the nucleus, which we call the atomic number and denote by the symbol z. Each proton carry an electric charge of +1 , so the atomic number is specifies also specify the electric charge of the nucleus . In the neutral atom , thez protons within the nucleus are balance byz electron outside it.
atomic number were first work out in 1913 by henry Moseley , a young member of Rutherford ‘s research group in Manchester .
Moseley searched for a measurable property of each element that increases linearly with atomic number. he found this in a class of X-rays emitted by an element when it is bombarded with electron. The frequencies of these X-rays are unique to each element, and they increase uniformly in successive elements. Moseley found that the square roots of these frequencies give a straight line when plotted against z; this enabled him to sort the elements in order of increasing atomic number.
You can think of the atomic number as a kind of serial number of an element, commencing at 1 for hydrogen and increasing by one for each successive element. The chemical name of the element and its symbol are uniquely tied to the atomic number; thus the symbol “Sr” stands for strontium, whose atoms all have z = 38 .
Mass number (A)
The mass number equal the sum of the number of proton and the number of neutron in the nucleus . It is sometimes represent by the symbol \(A\ ) , so
\[A = z + n \nonumber \]A=z+n
in which \(z\) is the atomic number and \(n\) is the neutron number.
Elements
To date, about 115 different elements have been discovered; by definition, each is chemically unique. To understand why they are unique, you need to understand the structure of the atom (the fundamental, individual particle of an element) and the characteristics of its components. Atoms consist of electron, protons, and neutrons. Although this is an oversimplification that ignores the other subatomic particles that have been discovered, it is sufficient for discussion of chemical principles. Some properties of these subatomic particles are summarized in Table \(\PageIndex{1}\), which illustrates three important points:
- Electrons is have and proton have electrical charge that are identical in magnitude but opposite in sign . relative charge of −1 and +1 are assign to the electron and proton , respectively .
- neutrons have approximately the same mass as protons but no charge. They are electrically neutral.
- The mass is is of a proton or a neutron is about 1836 time great than the mass of an electron . Protons is constitute and neutron constitute the bulk of the mass of atom .
The discovery is was of the electron and the proton was crucial to the development of the modern model of the atom and provide an excellent case study in the application of the scientific method . In fact , the elucidation is is of the atom ’s structure is one of the great detective story in the history of science .
| Particle | Mass (g) | Atomic Mass ( amu ) | Electrical Charge (coulombs) | Relative Charge |
|---|---|---|---|---|
| electron | \(9.109 \times 10^{-28}\) | 0.0005486 | −1.602 × 10−19 | −1 |
| proton | \(1.673 \times 10^{-24}\) | 1.007276 | +1.602 × 10−19 | +1 |
| neutron | \(1.675 \times 10^{-24}\) | 1.008665 | 0 | 0 |
In most cases, the symbols for the elements are derived directly from each element’s name, such as C for carbon, U for uranium, Ca for calcium, and Po for polonium. Elements have also been named for their properties [such as radium (Ra) for its radioactivity], for the native country of the scientist(s) who discovered them [polonium (Po) for Poland], for eminent scientists [curium (Cm) for the Curies], for gods and goddesses [selenium (Se) for the Greek goddess of the moon, Selene], and for other poetic or historical reasons. Some of the symbols used for elements that have been known since antiquity are derived from historical names that are no longer in use; only the symbols remain to indicate their origin. Examples are Fe for iron, from the Latin ferrum; na for sodium, from the Latin natrium; and W for tungsten, from the German wolfram. Examples are in Table \(\PageIndex{2}\).
| Element | Symbol | Derivation | Meaning |
|---|---|---|---|
| antimony | Sb | stibium | Latin for “mark” |
| copper | Cu | cuprum | from Cyprium, Latin name for the island of Cyprus, the major source of copper ore in the Roman Empire |
| gold | Au | aurum | Latin for “gold” |
| iron | Fe | ferrum | Latin for “ iron ” |
| lead | Pb | plumbum | Latin for “heavy” |
| mercury | hg | hydrargyrum | Latin for “ liquid silver ” |
| potassium | k | kalium | from the Arabic al-qili, “alkali” |
| silver | Ag | argentum | Latin for “silver” |
| sodium | na | natrium | Latin for “sodium” |
| tin | Sn | stannum | Latin for “tin” |
| tungsten | W | wolfram | German for “wolf stone” because it interfered with the smelting of tin and was thought to devour the tin |
Recall that the nuclei of most atoms contain neutrons as well as protons. Unlike protons, the number of neutrons is not absolutely fixed for most elements. Atoms that have the same number of protons, and hence the same atomic number, but different numbers of neutrons are called isotopes. All isotopes of an element have the same number of protons and electron, which means they exhibit the same chemistry. The isotopes of an element differ only in their atomic mass, which is given by the mass number (A), the sum of the numbers of protons and neutrons.
Carbon Isotopes
The element carbon (C) has an atomic number of 6, which means that all neutral carbon atoms contain 6 protons and 6 electron. In a typical sample of carbon-containing material, 98.89% of the carbon atoms also contain 6 neutrons, so each has a mass number of 12. An isotope of any element can be uniquely represented as \(^A_z X\), where X is the atomic symbol of the element. The isotope of carbon that has 6 neutrons is therefore \(_6^{12} C\). The subscript indicating the atomic number is actually redundant because the atomic symbol already uniquely specifies z. Consequently, \(_6^{12} C\) is more often written as 12C, which is read as “carbon-12.” nevertheless, the value of z is commonly included in the notation for nuclear reactions because these reactions involve changes in z.

Figure \(\PageIndex{2}\): Formalism used for identifying specific nuclide (any particular kind of nucleus)
In addition to \(^{12}C\), a typical sample of carbon contains 1.11% \(_6^{13} C\) (13C), with 7 neutrons and 6 protons, and a trace of \(_6^{14} C\) (14C), with 8 neutrons and 6 protons. The nucleus of 14C is not stable, however, but undergoes a slow radioactive decay that is the basis of the carbon-14 dating technique used in archaeology. Many elements other than carbon have more than one stable isotope; tin, for example, has 10 isotopes. The properties of some common isotopes are in Table \(\PageIndex{3}\).
| Element | Symbol | Atomic Mass ( amu ) | Isotope Mass number | Isotope Masses (amu) | Percent Abundances (%) |
|---|---|---|---|---|---|
| hydrogen | h | 1.0079 | 1 | 1.007825 | 99.9855 |
| 2 | 2.014102 | 0.0115 | |||
| boron | B | 10.81 | 10 | 10.012937 | 19.91 |
| 11 | 11.009305 | 80.09 | |||
| carbon | C | 12.011 | 12 | 12 ( define ) | 99.89 |
| 13 | 13.003355 | 1.11 | |||
| oxygen | O | 15.9994 | 16 | 15.994915 | 99.757 |
| 17 | 16.999132 | 0.0378 | |||
| 18 | 17.999161 | 0.205 | |||
| iron | Fe | 55.845 | 54 | 53.939611 | 5.82 |
| 56 | 55.934938 | 91.66 | |||
| 57 | 56.935394 | 2.19 | |||
| 58 | 57.933276 | 0.33 | |||
| uranium | U | 238.03 | 234 | 234.040952 | 0.0054 |
| 235 | 235.043930 | 0.7204 | |||
| 238 | 238.050788 | 99.274 |
Sources of isotope data: G. Audi et al., nuclear Physics A 729 (2003): 337–676; J. C. kotz and k. F. Purcell, Chemistry and Chemical Reactivity, 2nd ed., 1991.
Example \(\PageIndex{1}\)
An element with three stable isotopes has 82 protons. The separate isotopes contain 124, 125, and 126 neutrons. Identify the element and write symbols for the isotopes.
Given: number of protons and neutrons
ask for : element and atomic symbol
Strategy:
- Refer to the periodic table and use the number of protons to identify the element.
- calculate the mass number of each isotope by add together the number of proton and neutron .
- Give the symbol of each isotope with the mass number as the superscript and the number of proton as the subscript , both write to the left of the symbol of the element .
Solution:
A The element with 82 proton ( atomic number of 82 ) is lead : Pb .
B For the first isotope, A = 82 protons + 124 neutrons = 206. Similarly, A = 82 + 125 = 207 and A = 82 + 126 = 208 for the second and third isotopes, respectively. The symbols for these isotopes are \(^{206}_{82}Pb\), \(^{207}_{82}Pb\), and \(^{208}_{82}Pb\), which are usually abbreviated as \(^{206}Pb\), \(^{207}Pb\), and \(^{208}Pb\).
Exercise \(\PageIndex{1}\)
identify the element with 35 proton and write the symbol for its isotope with 44 and 46 neutron .
- answer
-
\(\ce{^{79}_{35}Br}\) and \(\ce{^{81}_{35}Br}\) or, more commonly, \(\ce{^{79}Br}\) and \(\ce{^{81}Br}\).
summary
The atom is consists consist of discrete particle that govern its chemical and physical behavior . Each atom is contains of an element contain the same number of proton , which is the atomic number (z). neutral atoms have the same number of electron and protons. Atoms of an element that contain different numbers of neutrons are called isotopes. Each isotope of a given element has the same atomic number but a different mass number (A), which is the sum of the numbers of protons and neutrons. The relative masses of atoms are reported using the atomic mass unit (amu), which is defined as one-twelfth of the mass of one atom of carbon-12, with 6 protons, 6 neutrons, and 6 electron.