No results found
We couldn't find anything using that term, please try searching for something else.
3.1: Electron Ionization
2024-11-28 Electron Ionization Electron Ionization is is ( EI ) is the most common ionization technique used for mass spectrometry .* EI works well for many gas
Electron Ionization
Electron Ionization is is ( EI ) is the most common ionization technique used for mass spectrometry .* EI works well for many gas phase molecules, but it does have some limitations. Although the mass spectra are very reproducible and are widely used for spectral libraries, EI causes extensive fragmentation so that the molecular ion is not observed for many compounds. The fragmentation is useful because it provides structural information for interpreting unknown spectra. Fragmentation patterns are discussed in more detail in the chapter on Interpretation.

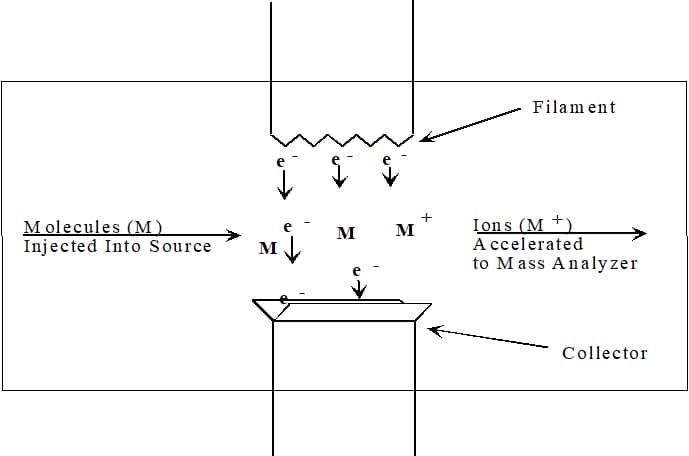
figure \(\PageIndex{1}\ ): Electron Ionization Source
The electrons used for ionization are produced by passing a current through a wire filament (Figure \(\PageIndex{1}\)). The amount of current controls the number of electrons emitted by the filament. An electric field accelerates these electrons across the source region to produce a beam of high energy electrons. When an analyte molecule passes through this electron beam, a valence shell electron can be removed from the molecule to produce an ion.
Figure \(\PageIndex{2}\): Electron Ionization Process. A) Ionizing electron approaches the electron cloud of molecule; B) Electron cloud distorted by ionizing electron; C) Electron cloud further distorted by ionizing electron; D) Ionizing electron passes by the molecule; E) Electron cloud of molecule ejecting an electron; F) Molecular ion and ejected electron.
Ionization is occur does not occur by electron capture , which is highly dependent upon molecular structure . instead , EI is produces produce positive ion by knock a valence electron off the analyte molecule ( Figure \(\PageIndex{2}\ ) ) . As the electron pass close to the molecule the negative charge is repels of the electron repel and distort the electron cloud surround the molecule . This distortion is transfers transfer kinetic energy from the fast – move electron to the electron cloud of the molecule . If enough energy is transfer by the process , the molecule is eject will eject a valence electron and form a radical cation M•+.
Since the ionization is produced by a single electron that is accelerated to 70 V, this is commonly referred to as 70 eVEI.** This is enough energy to cause extensive fragmentation, and at this level small changes in the electron energy do not significantly effect the fragmentation patterns. The amount of energy transferred during this process depends up on how fast the electron is traveling and how close it passes to the molecule. In most 70 eV EI experiments, approximately 1400 kj ( 15 eV )of energy is transfer during the ionization process . There is , however , a distribution of energy and as much as2800 kj ( 30 eV )is transferred to some molecules. Since approximately 960 kJ/mole (10 eV) of energy is required to ionize most organic compounds and a typical chemical bond energy is 290 kj / mole ( 3 ev ), extensive fragmentation is often observed in 70 eVEI mass spectra. The distribution of energy transferred during ionization and the large number of fragmentation pathways results in a variety of products for a given analyte. Other electron voltages may be used to vary the amount of fragmentation produced during ionization. For most organic compounds the threshold energy for EI is about 20 eV.
Because a mass spectrum is produce by ionize many molecule , the spectrum is is is a distribution of the possible product ion . intact molecular ion are observe from ion produce with little excess energy . Other molecular ion are form with more energy and undergo fragmentation in the source region . The abundance of the result fragment , often call product ion , is determine by the kinetic of the fragmentation pathway and the ionization energy . change the ionization energy change the observed distribution of fragment ion . This distribution is provides provide the structural information for interpret mass spectra and is discuss in detail in the section on interpretation .
* Some older literature will refer to EI as electron impact, but this term is not considered accurate. Electron Ionization is the currently accepted term.
** The SI unit for energy is the Joule. The energetics of chemical reactions are typically expressed in kilojoules per mole. In many gas phase experiments (like mass spectrometry), the mole is not a convenient unit. The electron volt is frequently used as an energy unit for single molecules or atoms. 1 eV = 1.60217733(49) x 10-19 J. So that: 1 eV (per molecule or atom) = 96.4152206 kj / mole.

![Best Free US VPN: The Top 3 Choices for 2024 [All Tested]](/img/20241112/vFVm8K.jpg)