No results found
We couldn't find anything using that term, please try searching for something else.
Electron Cloud Model
2024-11-25 Electron Cloud Modelpage 1 Slide 1 Ch. 10 - Atomic Structure II. Electron Cloud Model (p.272-274) Orbital Energy level Bohr Model Di
Electron Cloud Model
page
1
Slide 1
Ch. 10 – Atomic Structure
II. Electron Cloud Model
(p.272-274)
Orbital
Energy level
Bohr Model Diagrams
Slide 2

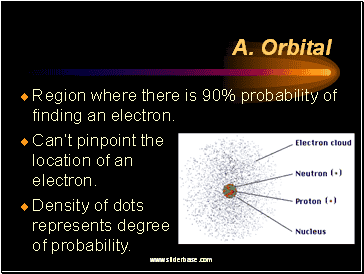
Orbital
Region where there is 90% probability of finding an electron.
Ca n’t pinpoint the location of an electron .
Density of dots represents degree of probability.
Slide 3
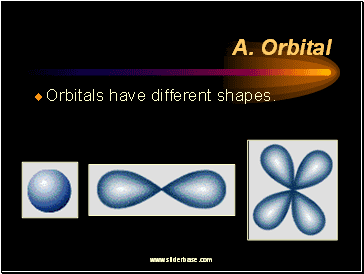
A. Orbital
Orbitals have different shapes.
Slide 4
Energy level
Electrons can only exist at certain energy levels.
Low energy levels are close to the nucleus.
Each energy level (n) can hold 2n2 electrons.
Slide 5
Bohr Model Diagrams
Simplified energy levels using Bohr’s idea of circular orbits.
e-
e-
Maximum e-
Level 1 2e-
level 2 8e-
level 3 18e-
Level 4 32e-
lithium
Atomic #: 3
Mass: 7
# of p: 3
# of e: 3
# of n: 4
e-
Slide 6
C. Bohr Model Activity
Choose a number between 1 & 18.
find your element by the atomic number you pick .
Draw a Bohr Model diagram for your element on your marker board.
Round off the mass listed on the table and subtract the atomic # to find the # of neutrons.
abbreviate the # of ‘ p ’ and ‘ n ’ in the nucleus .
Have a partner check your drawing.
repeat with a new element .


![How to Get a NordVPN Refund in 2024 [VPN for Free]](/img/20241121/fIOZE6.jpg)