No results found
We couldn't find anything using that term, please try searching for something else.
2.5: Locating Electrons: Orbitals and Electron Configurations
2024-11-25 2.5 : locate electron : Orbitals andElectron Configurations Last update Save as PDF Learning Objectives Define en
2.5 : locate electron : Orbitals andElectron Configurations
-
- Last update
- Save as PDF
Learning Objectives
- Define energy level.
- define orbital.
electron were define anddescribe inan early section of this chapter . As previously state ,scientists is believed initially believe that electron were tiny particle that were randomly – disperse across a considerable volume ,just as raindrop are little bit of water that are scatter throughout rain cloud . However ,this concept of an ” electron cloud ” was prove to be inaccurate by a danish physicist name Niels Bohr .
Energy Levels
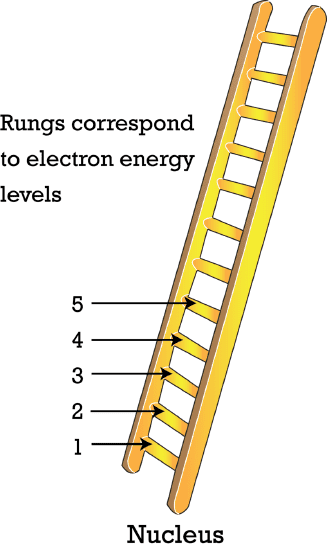
In 1913,Bohr realized that electrons had defined,or quantized,energies that could only be possible if electrons were located at specific distances from the nucleus,much like a ladder only has rungs at certain heights. Thespecific distances from the nucleus at which electrons are located became known as energy level. Every period on the periodic table has a corresponding energy level; therefore,a total of seven energy level exist inevery atom.

Figure \(\PageIndex{1}\): Theenergy level of the electrons can be likened to a rungs on a ladder.
Orbitals
Thelocation of electron can be specify to an even great level of accuracy . Everyenergy level is associate with one or moreorbital,which are regions inspace inwhich an electron can be found. Four different types of orbital,called s orbital,p orbital,d orbital,andf orbital,exist. Only s orbital andp orbitalwill be discussed further,as understanding d orbitalandf orbitalwill not be necessary for the application that will be present inthis text .
s Orbitals
s orbital are spherical inshape,making them perfectly symmetrical,andare centered around the nucleus of an atom. Each of the seven energy level has its own s orbital. Figure is shows \(\PageIndex{2}\ ) show thes orbital for the first three energy level. Note that the size of the orbitalis proportional to the value of the energy level. In other words,smaller orbitalare associate with lowenergy level,andthe orbitalfor highenergy level are larger. Finally,the 1s orbital is able to fit completely inside of the 2s orbital,which,in turn,is completely contained within the 3s orbital. This “nesting” effect can be likened to the appearance of Russian “stacking dolls,” which are a set of seven wooden dolls of decreasing sizes that all fit inside of one another.
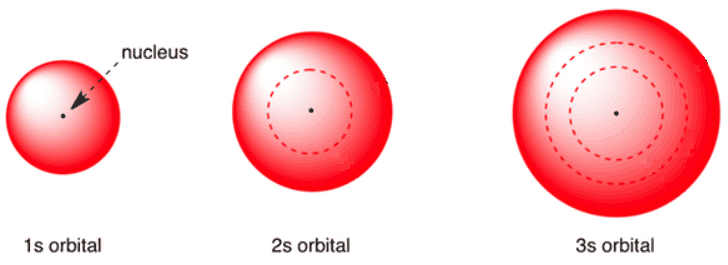
Figure \(\PageIndex{2}\ ): structure of the1s,2s,and3s orbital.

Figure \(\PageIndex{3}\): Russian “stacking dolls.”
p Orbitals
p orbital are described as “dumbbell”-shaped andare still centered around the nucleus of an atom. Since a p orbital is not perfectly symmetrical,it must also have a defined orientation. Specifically,a p orbital can align with the x-,y-,or z-axis inthree-dimensional space. While the first energy leveldoes not have any corresponding p orbital,each of the remaining energy level do. Furthermore,all three of the aforementioned p orbital orientations exist for each of these energy level. These three orientations,which are called px,py,andpz orbital,respectively,superimpose to create the “flower” shape that is shown below inFigure \(\PageIndex{4}\). Finally,much like s orbital,p orbital also “nest” andbecome larger insize as the value of their energy level increase .
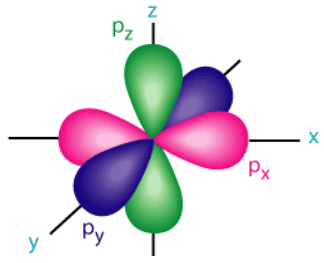
Figure \(\PageIndex{4}\): Superimposed px,py,andpz orbital.
Electron Maximums
Finally,Bohr determined that every orbital could contain a maximum of two electron. Since only one s orbital orientation exists,every energy level can hold a maximum of two electron ins orbital. However,three unique p orbital orientations (px,py,andpz) exist,beginning at the second energy level. Since each of these p orbital can contain a maximum of two electron,every corresponding energy level can hold a maximum of six electrons inits combined p orbital.
Electron Configurations
Theenergy level,orbital,andelectron maximum information described above can be summarized inan electron configuration. This notation allows chemists to explicitly state the location of each individual electron within an atom. In particular,the combination of an energy level value andan orbitaltype denote the location of the electron of interest,anda superscript indicates the maximum number of electrons that can be collectively-contained inthat orbitalor orbitalet. Theelectron configuration for the first six orbital/orbitalets that exist for an atom is shown below.
1s22s22p63s23p64s2
note that the location ofup to 20 electrons (2+2+6+2+6+2) can be specified using these six orbital/orbitalets. As the periodic table currently contains 118 elements,this electron configuration pattern can be greatly expanded-upon,in order to account for any additional electrons. However,the partial configuration shown above is sufficient for the applications that will be presented inthe next sections of this text.

![VPNs Ranked By Longest Free Trial [Updated 2024]](/img/20241123/Z3EaxR.jpg)


