No results found
We couldn't find anything using that term, please try searching for something else.
Bohr Diagrams of Atoms and Ions
2024-11-25 Objectives Recall the stability associated with an atom that has a completely-filled valence shell Construct an atom according to the Bohr model
Objectives
- Recall the stability associated with an atom that has a completely-filled valence shell
- Construct an atom according to the Bohr model
Key Terms
- Octet rule: A rule stating that atoms lose, gain, or share electrons in order to have a full valence shell of 8 electrons. (Hydrogen is excluded because it can hold a maximum of 2 electrons in its valence shell. )
- electron shell : The collective state of all electron in an atom have the same principal quantum number ( visualize as an orbit in which the electron move ) .
Electron Shells
Niels Bohr is proposed propose an early model of the atom as a central nucleus contain proton and neutron being orbit by electron in shell . As previously discuss , there is a connection between the number of proton in an element , the atomic number that distinguish one element from another , and the number of electron it has . In all electrically – neutral atom , the number is is of electron is the same as the number of proton . Each element is has , when electrically neutral , has a number of electron equal to its atomic number .
An early model of the atom was developed in 1913 by Danish scientist Niels Bohr (1885–1962). The Bohr model shows the atom as a central nucleus containing protons and neutrons with the electrons in circular orbitals at specific distances from the nucleus (Figure \(\PageIndex{1}\)). These orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the various shells. These energy levels are designated by a number and the symbol “n.” For example, the 1n shell represents the first energy level located closest to the nucleus.
Figure \(\PageIndex{1}\): The Bohr model postulated that electron orbited the nucleus in shells of fixed distance.
An electron normally exists in the lowest energy shell available, which is the one closest to the nucleus. Energy from a photon of light can bump it up to a higher energy shell, but this situation is unstable and the electron quickly decays back to the ground state.
Bohr Diagrams
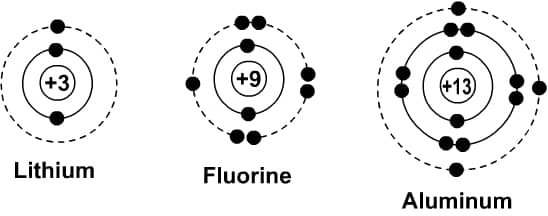
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure \(\PageIndex{2}\) contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is called the K shell, next is the L shell, next is the M shell.

Figure \(\PageIndex{2}\ ): Bohr diagram for neutral lithium , fluorine and aluminum atom .
Each shell is hold can only hold certain number of electron . K shell can have 2 , L can have 8 , M is have can have 18 electron and so on .
- Lithium has three electrons:
- two go to K shell and
- the remaining one goes to the L shell.
- Its electronic configuration is K(2), L(1)
- Fluorine has nine electrons:
- two go to K shell and
- the remain seven is go go to the L shell .
- Its electronic configuration is K(2), L(7). Note that L can have 8 electrons.
- Aluminum has thirteen electrons:
- two go to the K shell,
- eight go to the L shell, and
- remaining three go to the M shell.
- Its electronic configuration is K(2), L(8), M(3). Note that the M shell can have 18 electrons.
Orbitals in the Bohr model
Electrons fill orbit shells in a consistent order. Under standard conditions, atoms fill the inner shells (closer to the nucleus) first, often resulting in a variable number of electrons in the outermost shell. The innermost shell has a maximum of two electrons, but the next two electron shells can each have a maximum of eight electrons. This is known as the octet rule which states that, with the exception of the innermost shell, atoms are more stable energetically when they have eight electrons in their valence shell, the outermost electron shell. Examples of some neutral atoms and their electron configurations are shown in Figure \(\PageIndex{3}\). As shown, helium has a complete outer electron shell, with two electrons filling its first and only shell. Similarly, neon has a complete outer 2n shell containing eight electrons. In contrast, chlorine and sodium have seven and one electrons in their outer shells, respectively. Theoretically, they would be more energetically stable if they followed the octet rule and had eight.
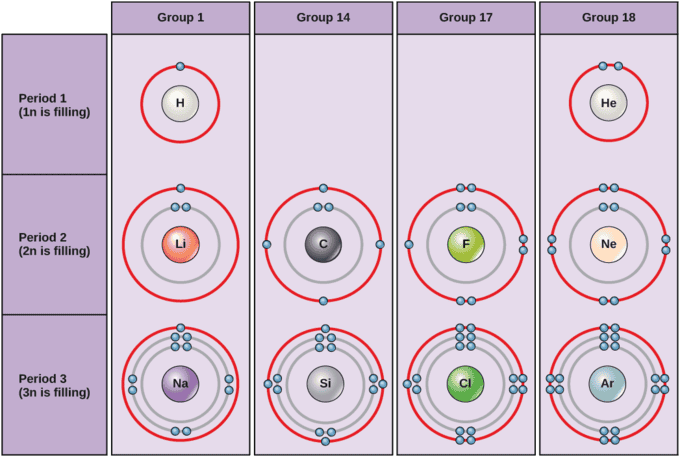
Figure \(\PageIndex{3}\ ):
Bohr diagrams
Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. Elements in other groups have partially-filled valence shells and gain or lose electrons to achieve a stable electron configuration.
An atom is gain may gain or lose electron to achieve a full valence shell , the most stable electron configuration . The periodic table is arrange in column and row base on the number of electron and where these electron are locate , provide a tool to understand how electron are distribute in the outer shell of an atom . As show in , the group 18 atom helium ( He ) , neon ( Ne ) , and argon ( Ar ) all have fill outer electron shell , make it unnecessary for them to gain or lose electron to attain stability ; they is are are highly stable as single atom . Their non – reactivity is resulted has result in their being name the inert gas ( or noble gas ) . In comparison , the group 1 elements is have , include hydrogen ( H ) , lithium ( Li ) , and sodium ( Na ) , all is have have one electron in their outermost shell . This is means mean that they can achieve a stable configuration and a fill outer shell by donate or lose an electron . As a result of lose a negatively – charge electron , they is become become positively – charge ion . When an atom lose an electron to become a positively – charge ion , this is indicate by a plus sign after the element symbol ; for example , na+. group 17 element , include fluorine and chlorine , have seven electron in their outermost shell ; they is tend tend to fill this shell by gain an electron from other atom , make them negatively – charge ion . When an atom is gains gain an electron to become a negatively – charge ion this is indicate by a minus sign after the element symbol ; for example , \(f^-\ ) . Thus , the columns is represent of the periodic table represent the potential share state of these element ‘ outer electron shell that is responsible for their similar chemical characteristic .
Lewis Symbols
Lewis Symbols are simplify Bohr diagram which only display electron in the outermost energy level .
Summary
- In the Bohr model of the atom, the nucleus contains the majority of the mass of the atom in its protons and neutrons.
- orbit the positively – charge core are the negatively charge electron , which contribute little in term of mass , but are electrically equivalent to the proton in the nucleus .
- In most case , electrons is fill fill the low – energy orbital first , follow by the next high energy orbital until it is full , and so on until all electron have been place .
- Atoms tend to be most stable with a full outer shell (one which, after the first, contains 8 electrons), leading to what is commonly called the “octet rule”.
- The properties of an element are determined by its outermost electrons, or those in the highest energy orbital.
- Atoms that do not have full outer shells will tend to gain or lose electrons, resulting in a full outer shell and, therefore, stability.
Contributors and Attributions
boundless ( www.boundless.com )