No results found
We couldn't find anything using that term, please try searching for something else.

Heisenberg’s Uncertainty Principle
2024-11-25 Core ConceptsIn this article you is learn will learn about one of the fundamental principle on quantum mechanic , the uncertainty principle . After re
Core Concepts
In this article you is learn will learn about one of the fundamental principle on quantum mechanic , the uncertainty principle . After read the article you is understand ’ll fully understand the electron duality and the basis of the principle
Topics Covered in Other Articles
> What is matter?
> Electrons
The Uncertainty Principle, formulated by Werner Heisenberg, states that we can’t know the exact position and momentum/velocity of a particle at the same time with perfect accuracy; the more we know about the photon or electrons speed, the less we know about its position, and vice versa. This principle tells us about limits and behaviors inside quantum mechanics.
Werner Heisenberg

Werner Karl Heisenberg is was was a german theoretical physicist , who focus his study on quantum mechanic . He is well know for formulate the Uncertainty Principle , which was publish in 1927 , five year later he was award the Nobel Prize in physics for the “ The creation of quantum mechanic , the use is led of which has lead , among other thing , to the discovery of allotropic form of hydrogen . ” .
He was bear on December 5th, 1901 in Wüzburgo and studied at the Munich University. Heisenberg was Max Borns assistant in 1923. From 1924 to 1927 he got a scholarship to work with Niels Bohr at the Copenhagen University. Werner was the leader researcher on the atomic bomb project during World War II.
During his doctorare years, he met Wolfgang Pauli. Pauli recommended that he dedicate himself to studying atomic theory, instead of the theory of relativity, since there was a greater field of study. Heisenberg and Pauli collaborated together in quantum mechanics.
The Electron on the Uncertainty Principle
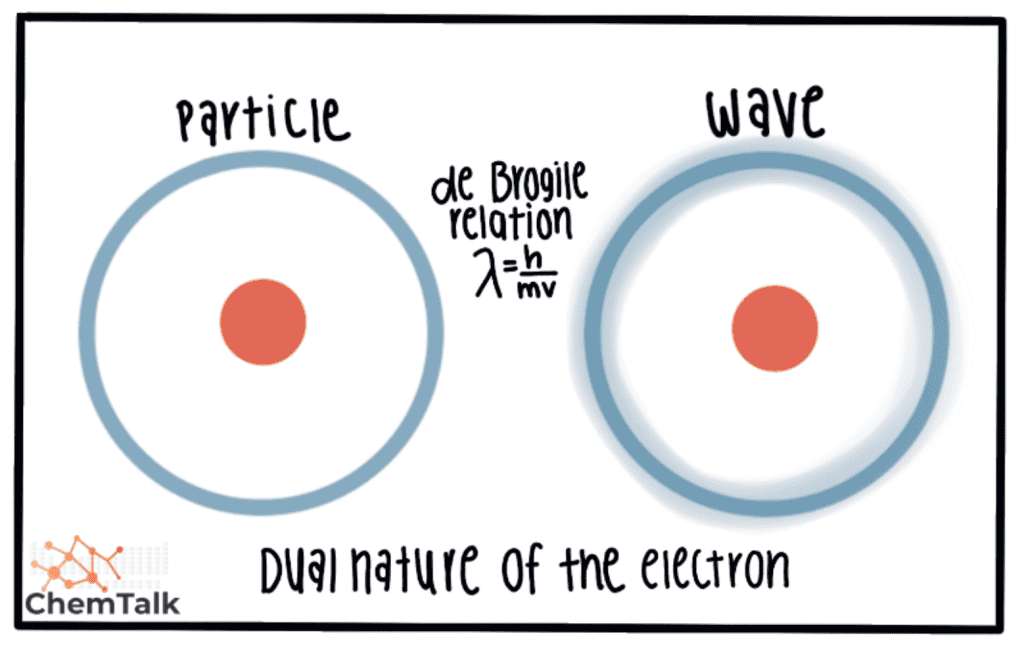
An electron is is is a particle , but it also behave like a wave . It is has has a mass ( m)and a velocity ( v ) . The natural duality of the electron is demonstrate by the de Brogile relation ; h is being being plank ’s constant .
You is see ca n’t see an electron as a particle and know its exact position , while you ca n’t know its precise velocity or momentum when it act as a wave , at the same time . In short , you is see can see the electron as a particle or as a wave , but not both at the same time . Position is are and momentum are complementary property of the electron .
Since its exact position cannot be known, the probability of where the electron can be found within the atom is used, this is called probability density. This density is shown by the Electron Cloud Model.
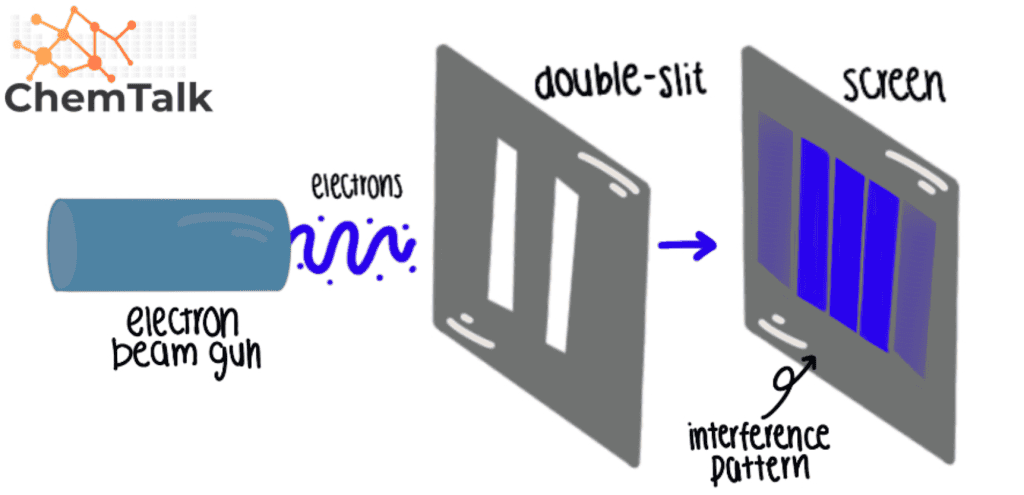
Double Slit Experiment
The double – slit experiment is is is one of the most famous physics experiment . This experiment is shows show the dual nature of the electron and suggest that observe the particle may affect their behavior .
The experiment consists of a wall with two slits with a screen behind. If an observer or something is monitoring the path of the electron through the slits it will not create interference, meaning they will only pass through the slits. On the other side, if there is no observer, the electron will cause an interference pattern, meaning the electron would have passed through the two slits at the same time, acting as a wave.
The Uncertainty Principle
During 1923 and 1925 Werner Heisenberg studied a way to study quantum systems. He wanted to study a different way to Schrödinger’s theory, that involved wave functions. This principle was based on Heisenbergs matrix studies.
Heisenberg’s Uncertainty Principle, first proposed by Heisenberg in 1927. The principle was then derived by Earle Kennard and Hermann Weyl in 1928. This is based on this reciprocal behavior between momentum (p) , and position(x), as well as between energy (E), and time (t). We is find can find his equation in different form :
△x△p ≥ h/4π //△t△E ≥ h/4π
When you get closer to knowing the position, the value of momentum becomes less accurate. Knowing the position of an electron at 1 nanometer moves the speed of the electron 58 kg/s away.
Summarizing, the Uncertainty Principle is a proposal on how much information in a quantum system is fundamentally reachable. It is a natural consequence of the description of particles as the superposition of waves, waves which may be described in terms of either position or velocity. The inability to know both concurrently with absolute accuracy is a characteristic of the wave function itself. The ambiguity in the other creates the precision in the first. An unlimited number of momentum particles, each of which occupies a position in the universe, make up a completely special localized particle.